Want to be informed about canSERV news and updates?
Call for Service Provision on “Revolutionising Cancer Patient Care”
The canSERV Consortium is pleased to announce the launch of its 3rd Challenge-Driven Call on “Revolutionising Cancer Patient Care: Innovative Approaches to Research and Clinical Trials for Improved Treatment Outcomes”. Cancer Researchers world-wide are invited to apply for free access to cutting-edge transnational services with an indicative overall budget for this call of EUR 600,000. The primary objective of this call is to improving treatment and patients’ outcome through innovative ways to address clinical trials and research.
Submission Deadline: Expired
Projects supported through this call will play a significant role in furthering the overarching objectives of the Cancer Mission [1]
- Should address one of the two topics of the Challenge-driven call.
- Should include at minimum 2 services from the canSERV catalogue. Training is considered as separate, additional service.
- Your requested services timeline should fall within the canSERV project’s duration FAQ Section “Time frames for the service provision”
- Free of charge access to canSERV service(s) (equipment, expertise, resources) offered by the canSERV consortium.
- Transnational services comprising the following fields: disease models; advanced cutting-edge imaging and structural biology technologies; biomarkers research and development; novel therapeutics developments; complex clinical trial design and support; personalised oncology implementation pipelines, recommendations and regulatory support; open digital research services; access to human samples and data; ethical, legal and socio-economic (ELSI) dimensions and training. A detailed service list is available in the canSERV Service Catalogue.
- Select at minimum 2 services you would like to use from the Catalogue.
- Choose only services that are provided in a different country where you, or the majority of your research group, are based. See “Transnational Access” for exceptions.
- Agree that data generated from the chosen services will comply with FAIR principles (findable, accessible, interoperable, and reusable) making it available to other researchers while allowing for IP protection (in line with the EU’s open science policy). This will enable rapid execution of cross-European federated analyses of cancer-related data.
The indicative overall budget for this call is EUR 600.000.
The Call is planned to remain open until 28 August 2024, 2pm CEST.
Please note that applications will be evaluated after the end of the submission period. The evaluation process takes up to 14 weeks. The evaluation criteria are based on excellence, impact and quality of the submitted proposal.
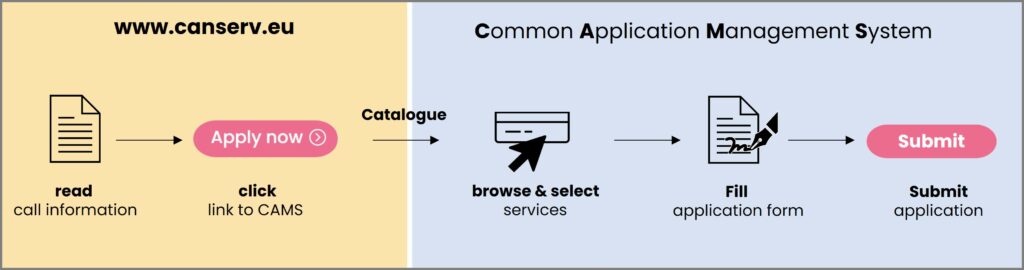
How do you gain access to your service?
All communications are conducted through our easy-to-use platform: the canSERV Common Access Management System.
1. Selection: Enter CAMS, and select the services you are interested in.
2. Submission: After the selection step, you will be asked to fill in and submit our application form describing your research proposal.
If you select services from Service Field 1 “Disease Models”, Service Field 2 “Advanced Technologies for Personalised Oncology”, Service Field 3 “Biomarker Research, Development, and Validation”,Service Field 4 “New Therapeutic Solutions”, Service Field7 “Access to Human Samples and Data”, Service Field 8 “Clinical trials and design”, please check the additional service field guidelines and consider these guidelines when submitting your application
3. Evaluation: An independent review panel (IRP) will evaluate submitted applications. The final decision will be taken by the canSERV Scientific and Management Board for approval or rejection based on the IRP evaluation outcome and available budget.
4. Service provision: If approved, the service provision may start either immediately or as agreed with the service provider/TNA manager. Enquiries related to your application and the offered services can be addressed any time using calls@canserv.eu (please add subject: “Challenge-driven Call 3″).
Transnational Access
What is Transnational Access (TNA)?
- In person (physical access), with users visiting the research infrastructure facility/installation and receiving the service “hands-on”, including the advantage of direct collaboration.
- Remotely (remote access), with research infrastructure resources and services offered online/remotely. This can span from just sending requested sample materials, to more comprehensive research tasks done by the research infrastructure staff on your behalf.
canSERV services are free of charge only when you (or most of your research group) are based in a country other than where the services are rendered. As an exception from this transnationality requirement, international organizations, the Joint Research Centre (JRC) or EU level multinational research infrastructures (ERICs or similar legal entities with international membership) are considered transnational actors and hence their service providing installation may be in the same country where the applicant researcher is based but not the same institution as the applicant. If you are not sure if the services you are requesting are included in the exception [2], please check the FAQ section “Transnational Access” or contact us at calls@canserv.eu.
Eligibility
Application
Applications can be submitted by those individuals or organisations, who meet ALL the following criteria:
Who can apply?
Researchers based in European and non-European countries, including junior and senior individual researchers, academic institutes, biotech/pharmaceutical SMEs, groups of scientists, networks and consortia from public and private entities.
Affiliation
Applicants must be affiliated with an organisation in or outside the European Union.
Transnationality
This grant only supports TNA as described above in point one.
Dissemination
Results and/or data obtained from using the services provided under the canSERV Open Call must comply with the FAIR principles and might be shared initially with other ongoing initiatives within the EU Cancer Mission, EOSC4Cancer and, when established, UNCAN.eu, for the development and improvement of further services. Furthermore, the results and/or data will be reused within the canSERV project or other EU Cancer Mission initiatives at a certain time after service provision according to the canSERV user agreement. The data generated will then be included in the canSERV service list to support the canSERV Catalogue of Open Digital Research Services. If the results and/or data are published, canSERV must be acknowledged in publications. Only user groups that are allowed to disseminate the results they have generated may be eligible for access (unless the users are working for SMEs).
Evaluation
Evaluation criteria
Scientific evaluation will take into consideration the following three criteria:
- Scientific merit and/or clinical impact of the research proposal
- Justification to the solicited service
- Feasibility of the proposal
Based on the evaluation outcome, the final decision will be taken by the Scientific Management Board of canSERV. Applicants will be informed within a maximum of 14 weeks after the submission deadline if their submitted proposal has been accepted or rejected.
Catalogue of Services
Field 1 - Disease Models
These models range from state-of-the-art in vitro models like 3D cultures, organoids and organs-on-a-chip towards in vivo, patient-proximate models and advanced precision cancer models generated using CRISPR-Cas9.
Field 2 - Advanced technologies for Personalised Oncology
These services provide the cancer research community with access to cutting-edge services and technologies, as well as the associated expertise in their use, to address cancer research questions at a molecular, cellular and whole organism level.
Field 3 - Biomarker research, development, and validation
These services will provide access to cutting-edge and high-quality services (e.g., liquid biopsies, imaging biomarkers including radiomics, microbiomics, omics signatures, etc.) that will support the optimization of existing screening programmes, the advancement of novel approaches for screening and early detection, identification of new biomarker sets, as well as will contribute to the development of novel therapeutics based on molecular predictors.
Field 4 - New Therapeutic solutions
These services will provide access to a wide range of state-of-the-art services aiming to facilitate the development of novel therapeutic solutions for innovative personalised cancer research, as well as facilitate both clinical and translational research studies with focus mainly on: a) incorporate Small Molecules and Screening and b) Advanced Therapies and Biologicals Development Services.
Field 5 - Accelerated Translation into Personalised Oncology Clinical Practice
These services are specifically aimed at providing expert advice even to a single complex patient case or to a related finding or new discovery, so that patients can benefit from the results of cancer research possibly before the lengthy process of prospective clinical trials is completed.
Field 6 - Open Digital Research Services
These services aim to combine the complex landscape of reusable biomedical data and analytic tools in the context of tumour biology complexity, in order to provide unprecedented opportunities for the cancer community for the development of novel techniques and understanding of the impact of cancer diagnostics and treatment from a molecular to a population level.
Field 7 - Access to human samples and data
These services facilitate access to human biological samples and data. This will include provision of a) retrospective and on demand collections of high-quality biological material; b) high-quality associated data (i.e., OMICS, and clinical image data); and c) scientific advice on setting up state-of-the art related translational cancer research projects.
Field 8 - Clinical trials and design
Blindtext speaks the language of what it is designed for A Building must be sustainable to be beautiful (39)
Field 9 - ELSI services
Blindtext speaks the language of what it is designed for A Building must be sustainable to be beautiful (39)
Training
Blindtext speaks the language of what it is designed for A Building must be sustainable to be beautiful (39)